The Era of Personalized Medicine Has Arrived – PMC’s Annual Progress and Outlook Report

The Personalized Medicine Coalition (PMC) released its annual “Personalized Medicine At FDA: A Progress & Outlook Report” (Report) that monitors current successes and challenges in bringing personalized therapies to market.
Notable Developments for 2018
Several milestones were noted for 2018 – ranging from new drug approvals to direct-to-consumer genetic testing.
1. A record number of personalized medicine approvals (42% of all 2018 new drug approvals) by FDA. Twenty-five of the 59 new molecular entities FDA approved in 2018 were personalized therapies. Over 30% of all new drug approvals were personalized medicines. PMC defines a personalized therapy as any treatment that uses a diagnostic test to determine which treatment is best for each patient.
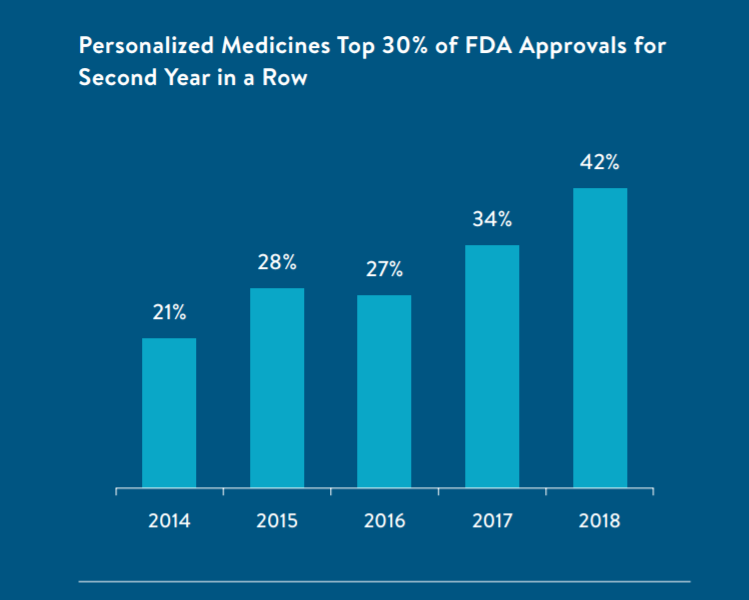 Report at Page 5.
Report at Page 5.
2. FDA’s approved the cancer drug Vitrakvi (larotrectinib) for the treatment of all solid tumors that express neurotrophic receptor tyrosine kinase (NTRK) gene fusion, wherein the use of the drug is based on the presence of the NTRK biomarker rather than the tumor type or tissue of origin of the tumor. This is the second approval of a cancer drug indication based on the presence of a biomarker. Report at pages 2 and 9.
3. FDA’s first approval of a siRNA (small interfering ribonucleic acid) drug for the treatment of polyneuropathy of hereditary transthyretin-mediated amyloidosis (based on the presence of the TTR biomarker status in patients). Report at page 9.
4. FDA authorized the first direct-to-consumer pharmacogenetic and cancer risk-related genetic tests. FDA’s approval of 23andMe’s limited BRCA variant test for breast and ovarian cancer risk is the first available approved test for cancer risk without a prescription. FDA also authorized 23andMe’s Personal Genome Service Pharmacogenetic Reports that provide information about 33 genetic variants that may be associated with a patient’s ability to metabolize some medications. Report at page 13.
5. FDA recognized a public human genetic variant database to support claimed relationships between tested genetic variants and disease: the Clinical Genome Resource (ClinGen) database. The use of the public databases is reported to increase the use of real-world data for oversight purposes and reduce clinical development burden associated with the time and cost of developing personalized medicine diagnostics. Report at page 13.
6. FDA approved Truxima (rituximab-abbs) and Herzuma (trastuzumab-pkrb); two new biosimilars for existing personalized medicines. Truxima is a Rituxan biosimilar and Herzuma is a Herceptin biosimilar. Report at page 10.
Remaining Challenges
According to the PMC, several challenges remain that could limit future progress. Predictable and sufficient funding must exist. The PMC urges policy makers to favor policies that encourage the advancement of the field as they have in the past. This includes a “predictable funding environment for FDA’s activities … to overcome reimbursement challenges associated with personalized tests and treatments that in turn increased up-front costs into downstream benefits for patients and health systems.” Report at page 16.
An additional stressor is the lack of resources at FDA to prioritize the development of innovative personalized medicine products and services. As noted by the PMC – “Investors will only continue to prioritize the development of innovative personalized medicines … for as long as they are reasonably certain that FDA, which serves as a gatekeeper for the products that reach patients, will have the resources necessary to complete its work in a timely manner. Any uncertainty in relation to the future of agency funding has the potential to disrupt highly sensitive investment markets.” Report at page 16.
Maintaining Progress
The approval of a large percentage of personalized medicine therapies by FDA indicates that innovation is moving toward more customized, effective and safe therapies. While challenges remain, PMC’s Report concludes that: “The era of personalized medicine has arrived…. [T]he science, more than ever, is leading the health system away from one-size-fits-all, trial-and-error medicine and toward the utilization of molecular information to improve outcomes and make the health system more efficient.” Report at page 18.




