Personalized medicine selects the best therapy or course of treatment for each patient based on diagnostic testing. In one aspect, personalized medicine uses pharmacogenetic testing to provide information regarding: 1) how an individual’s genes affect that person’s response to medications; 2) the best dose of a medication; and/or 3) whether the individual could suffer serious side effects from a medication.i
Pharmacogenetic testing is currently being used to select therapies to treat a wide range of medical conditions including cardiac disease, depression and cancer:ii
CDRH and CDER Collaborative Review
Recently FDA stated that pharmacogentic testing is useful when there is sufficient scientific evidence demonstrating a relationship between a patient’s genotype and the healthcare outcome. However, such evidence does not exist for many medications. FDA raised particular safety concerns with companies offering genetic tests and “making claims about how to use the genetic test results to manage medication treatment that are not supported by recommendations in the FDA-approved drug labeling or other scientific evidence.” FDA Announces Collaborative Review of Scientific Evidence to Support Associations Between Genetic Information and Specific Medications” (“Announcement”) .
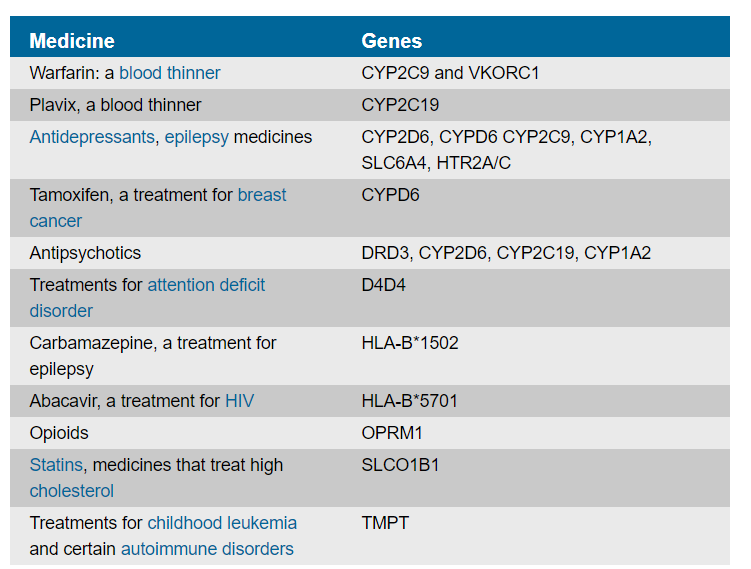
On February 20, 2020, FDA announced a collaboration between the FDA’s Center for Devices and Radiological Health (“CDRH”) and Center for Drug Evaluation and Research (“CDER”) to provide FDA’s view of the state of current science in pharmacogenetics. Id. The agency also published a table that describes some of the gene-drug interactions for which the agency believes there is sufficient evidence to support the described associations between certain genetic variants and one or more of altered drug metabolism, differential therapeutic effects, and/or differences in risks of adverse events. Id. While not complete, the table should provide clinicians with important information to evaluate currently available genetic tests while the agency continues to review the scientific evidence.
FDA also opened a docket for public comment to solicit feedback on specific pharmacogenetic associations that should or should not be included as FDA continues to update the table.
The agency also announced that it is exploring additional approaches for evaluating the evidence underlying gene-drug interactions such as community-based approaches.
FDA’s Commitment to Personalized Medicine
Safety and efficacy of personalized medicines is important to the continued success and adoption of personalized medicine. The FDA continues to signal the agency’s support of personalized medicine. We will continue to monitor FDA’s oversight of the industry’s use of genetic testing. Companies that market such tests should continue to be careful about medical claims in their promotional materials for personalized medical treatment or tests, especially when such tests serve the direct-to-consumer market.
ii Reproduced from https://medlineplus.gov/lab-tests/pharmacogenetic-tests/
