In In re Cellect, the Federal Circuit effectively held that Patent Term Adjustment (PTA) awarded under 35 USC § 154 is not protected from obviousness-type double patenting (OTDP) in view of a patent with the same 20-year term, such as may arise between a parent and child patent. In so doing, the court drew a line between other Federal Circuit decisions holding that Patent Term Extension (PTE) awarded under 35 USC § 156 is protected from OTDP. This decision may lead stakeholders to reconsider patent strategies that include routinely obtaining continuation patents, especially after a parent patent has been awarded a significant amount of PTA.
The OTDP Issues
The decision arose from four ex parte reexamination proceedings in which the USPTO Patent Trial and Appeal Board held certain claims of U.S. Patent 6,982,742, U.S. Patent 6,424,369, U.S. Patent 6,452,626, and U.S. Patent 7,002,621 invalid for OTDP. The patents all stemmed from the same priority application, but had different expiration dates due to different PTA awards.
This diagram from the Federal Circuit decision explains the relationships among the patents:
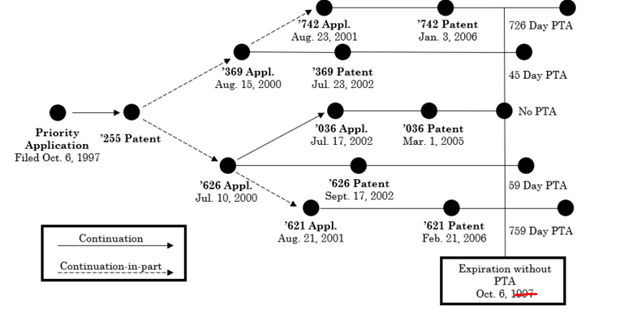
(As stated elsewhere in the decision, the “Expiration without PTA” was October 6, 2017)
Note that by the time of the reexamination proceedings, the patents had expired. Nevertheless, the patentee was interested in pursuing damages for past infringement.
This chart from the Federal Circuit decision explains the OTDP findings:
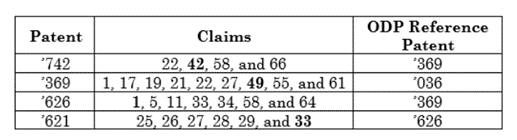
Note that the ’036 patent earned no PTA and the ’369 patent earned less PTA than the other patents.
Cellect did not challenge the “obviousness” aspects of the OTDP findings, instead relying on legal and equitable arguments why the differences in patent term due to the different PTA awards should not give rise to OTDP.
The Federal Circuit Decision
The Federal Circuit decision was authored by Judge Lourie and joined by Judges Dyk and Reyna.
Most of the decision explains why the holdings in Merck & Co. v. Hi-Tech Pharmacal Co. (Fed. Cir. 2007) and Novartis AG v. Ezra Ventures LLC (Fed. Cir. 2018), which addressed the interplay between Patent Term Extension (“PTE”) awarded under 35 USC § 156 and OTDP do not apply to PTA awarded under 35 USC § 154. For example, the court found that § 154 itself indicates that PTA is subordinate to OTDP, by providing that PTA cannot extend a patent beyond an expiration date specified by a terminal disclaimer (which may be filed to overcome an OTDP rejection or resolve an OTDP issue post-grant).
The court also addressed Cellect’s arguments that because PTA is a statutory award intended to compensate for USPTO examination delays, it cannot give rise to “an unjustified timewise extension of patent term,” or raise the gamesmanship concerns the judicially-created OTDP doctrine aims to address. The court explained that, if OTDP did not apply, the later-expiring patents would in effect confer PTA on the earliest-expiring patent that it did not earn—i.e., resulting in an unjustified extension of patent term of the earliest-expiring patent.
Cellect Strategies
Some stakeholders have been expecting a decision like Cellect since the Federal Circuit first decided that a later-granted patent can raise OTDP issues for an earlier-granted patent, in its 2014 decision in Gilead Sciences, Inc. v. Natco Pharma Limited. Others thought the Novartis v. Ezra case raised the possibility that the Federal Circuit would protect PTA from OTDP. Now that the Federal Circuit has rendered this decision, stakeholders may want to reconsider patent strategies that include routinely obtaining continuation patents. For example, stakeholders could consider on a case-by-case basis whether the additional scope of protection offered by a continuation patent is worth sacrificing any PTA awarded to a parent patent, or consider if a similar scope of protection could be provided by a divisional application. For stakeholders with granted patents retroactively impacted by Cellect, the decision notes that terminal disclaimers “may be filed to overcome an ODP rejection assuming that the first patent has not yet expired.” Thus, it may be possible to cure invalidity issues raised by this decision by filing a terminal disclaimer. On the other hand, since OTDP arises on a claim-by-claim basis, stakeholders may want to proceed carefully if only some claims are vulnerable to an OTDP challenge.
