The USPTO has released an updated report on Inter Partes Review (IPR) and Post Grant Review (PGR) proceedings involving Orange Book or biologic patents, taking into account data through March 31, 2023. Highlights of the report that might be of interest to stakeholders in this space include trends in differences in numbers of petitions, institution rates, and outcomes as between Orange Book-listed patents and biologic patents. While the Hatch-Waxman ANDA litigation paradigm may favor district court litigation over PTAB trials for Orange Book-listed patents, it is interesting to consider possible reasons behind other differences.
The images in this article include excerpts from USPTO presentation available here.
Trends in Number of AIA Petitions
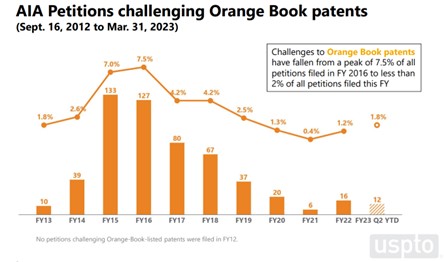
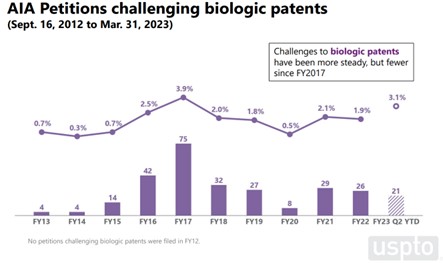
The number of AIA petitions challenging Orange Book and biologic patents, which account for 4% and 2% of all AIA petitions, respectively, has dropped since its peak in 2016-2017. The drop in AIA petitions is more pronounced for Orange Book patents, which has gone from 7.5% of all petitions filed in FY 2016 to less than 2% of all petitions filed this year (a more than 80% drop). The number of AIA petitions challenging biologic patents has been steadier since its 3.9% peak in 2017, having climbed back up to near its peak levels in FY 2023 (3.1% by Q2) after a steady low of about 2% between FY 2018 to FY 2022.
Trends in Trial Type
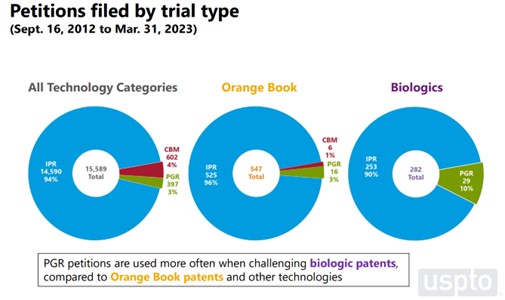
While the total number of PGR petitions remains low, the USPTO’s statistics indicates that biologic patents are the most likely to be challenged in a PGR proceeding. To date, biologic patents have been the subject of about 90% of all PGR petitions filed. Perhaps the technology of biologic patents makes them more subject to challenge on the written description and enablement grounds that only can be raised in a PGR proceeding.
Trends in Institution Rates
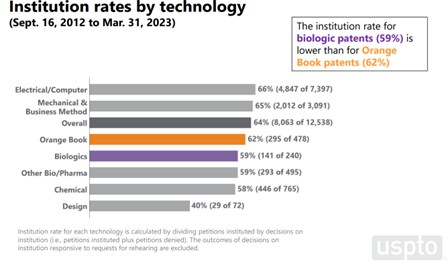
The overall institution rate for Orange Book patents (62%) is slightly higher than the rate for biologic patents (59%); however, the difference may not be statistically significant. The USPTO data for Orange Book patents are based on 478 petitions (295 institutions), while the data for biologic patents are based on 240 petitions (141 institutions). The institution rate for all petitions is 64%, based on 12,538 petitions (8063 institutions).
Trends in Outcomes
There are some differences in outcomes between Orange Book patents and biologic patents. The per-patent data indicate that 19% of Orange Book patents survive challenge with all claims patentable, while only 7% of biologic patents fare the same. On a claim-by-claim basis, 17% of challenged Orange Book claims (33% of instituted claims) were found unpatentable, while 25% of challenged biologic claims (57% of instituted claims) were found unpatentable.
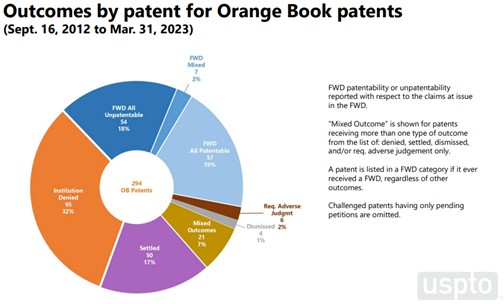
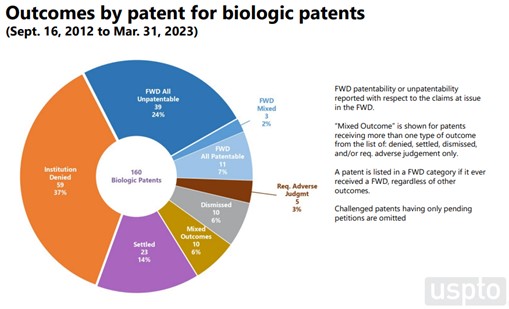
Note the “Outcomes” data set is smaller than the “Institution” data set, because it is limited to completed proceedings.
Do The Trends Tell A Tale?
On the surface, the USPTO’s data suggest that biologic patents have a slightly better chance of avoiding institution of an IPR or PGR proceeding, but if a proceeding is instituted, biologic patents have a greater chance of having claims invalidated. Does that mean Orange Book patents are “stronger” than biologic patents?
One thing to keep in mind when reviewing these statistics is that the data set for Orange Book patents is more limited than the data set for biologic patents. As explained in the 2019 version of the report, to be counted as an Orange Book patent, the patent had to be listed in the FDA’s electronic Orange Book when the IPR or PGR petition was filed. On the other hand, the PTAB manually identified biologic patents “as any patent potentially covering a Purple Book-listed product and any non-Orange Book-listed patent directed to treating a disease or condition.” Thus, where Orange Book patent owners already identified the patents as pertaining to an approved pharmaceutical product, no such patent owner selection was a prerequisite for being considered as a biologic patent.
Special thanks to Meyke Kang, a summer associate in Foley’s Washington, D.C. office, for her contributions to this article.
